
This editorial appears in the April 2024 issue of the American Journal of Bioethics.
Informed consent documents are central to the informed consent process and are required for participation in clinical trials in the U.S. The primary purpose of the document is “to assist a prospective subject” in deciding to participate in a research study by (1) clearly presenting the necessary information “not merely provide lists of isolated facts,” and (2) to “facilitate comprehension.” However, over time these documents have become increasingly complex and burdensome to create, update, review, read, and comprehend for all stakeholders. Moreover they pose an obstacle to clinical trial enrollment, especially among underserved minority populations.
There is dissatisfaction among all the stakeholders invested in drug development, including IRBs, physicians, clinical trial sponsors, research participants, and regulators, with respect to the current state of informed consent documents. The documents are excessively long, the list of mandatory items running to more than 270 words. Indeed over the last two decades, industry-wide, they have gone from being three to four pages to commonly being over twenty pages. They are often written at too high a reading level and have become increasingly legalistic, in order to comply with legislation e.g. data privacy, and after having been used as critical documents in lawsuits. Unsurprisingly, one study found that the majority of U.S. adults would struggle to comprehend consent documents. And yet, there has not been a coordinated effort, across all stakeholders, to drive substantial improvement. As a result, consent documents only continue to grow in length and complexity.
A worthy goal is to return informed consent documents to their proper role. To that end, Janssen R&D and the University of Pennsylvania are working together to shorten informed consent documents, and improve readability and comprehension by removing unnecessary language, eliminating lists, and minimizing the use of long words and technical language while ensuring that critical ethical and legal requirements are maintained.
Illustrative of this effort is a revision of the risk information, probably the most important part of informed consent. When potential participants are considering enrollment in a clinical trial and are reviewing a consent document, they have not been assigned to an intervention group. They are concerned about the likelihood and magnitude of potential side effects, and how these will be mitigated or treated. Consent documents typically list all of the drugs in a regimen and their associated risks separately and describe the different trial arms, leaving it to participants to integrate the information on likelihood, severity, timing, and treatment of side effects on their own. Our proposed revision eliminates the exhaustive list of potential side effects from each individual drug or procedure and instead combines the risks into a summary table. Aggregating risks for each trial arm in a table that also provides information about frequency can be done in an efficient and comprehensive fashion (Table 1). Using the available information to date (e.g. from the Investigator’s Brochure), potential side effects can be presented in tabular form, based on their severity, and time of onset and their frequency (Table 2). This should make it easier for patients to assess overall risks regarding what they can expect across the different treatment regimens. In one example of an ongoing chemotherapy trial for multiple myeloma, an aggregated, tabular presentation of risks in 1,113 words replaced a drafted risk section of over 4,500 words.

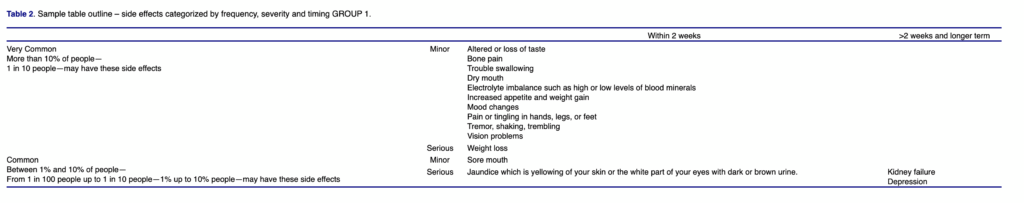
That such a tabular presentation of risk data rather than lists of side effects improves comprehension is a hypothesis. Janssen R&D is collecting data to evaluate study comprehension, assess participants’ satisfaction with the tabular format, enrollment, and retention rates, and obtain stakeholder feedback on the modified ICF as compared to the typical documents used in current clinical trials.
This is a small step on the path to streamline and enhance the usability of informed consent documents. Only empirical research can determine which steps ensure documents are better at providing potential research participants necessary information to facilitate their participation—or informed refusal—in oncology clinical trials.
At present, ideas about how consent documents should be written are based on intuition and opinion rather than evidence. Little data exists on the most effective methods of presenting information in consent documents. A data-driven approach to improving consent documents will require support and input from IRBs and regulatory agencies. IRBs can play a major role in this effort by facilitating empirical research to determine how to ensure prospective research participants are well informed by ICFs. In addition, IRBs and regulatory agencies should closely collaborate with clinical research sponsors, investigators, and patient advocates to support approaches that prioritize comprehension of informed consent documents over length and complexity.
Shortening and improving the communication of information in informed consent documents may also help improve access to clinical research. Industry-wide, significant effort is being made to improve the diversity of clinical trial populations, by enrolling groups that have traditionally been underrepresented. It will be important to empirically assess whether shorter, more readable documents that focus on information that can be more readily comprehended by potential study participants will enhance recruitment of lower socioeconomic, nonwhite populations. But only research will tell.
Disclosure Statement
Tendler C, Kane C, Kopaczynski C, and Terry W are employees of Janssen Research & Development, Pharmaceutical Companies of Johnson & Johnson and own stock in Johnson & Johnson. Emanuel E reports speaker honoraria from The Galien Foundation, WellSky, RightWay, Signature Healthcare Foundation, Healthcare Leaders of NY, Medimpact, Massachusetts Association of Health Plans, Philadelphia Committee on Foreign Relations, Yale University, Hartford Medical Society, AAHC Global Innovation Forum, HMSA & Queens Health System, Advocate Aurora Health Summit, University of Pittsburgh Medical Center (UPMC) Shadyside, University of California San Francisco, Advocate Aurora Health, Cain Brothers Conference, Bowdoin College, The Suntory Foundation, Ontario Hospital Association, University of Oklahoma; visiting professor honorarium from Princeton University; support for attending meetings and/or travel from Hartford Medical Society, AAHC Global Innovation Forum, Macalester College, Oak CEO Summit, Advocate Aurora Health Summit, DPharm Conference, UPMC Shadyside, UCSF San Francisco, Cain Brothers Conference, Bowdoin College, Galien Jerusalem Ethics Forum, HLTH 2022 Las Vegas, HMSA Honolulu, Tel Aviv University, The Suntory Foundation, The Ontario Hospital Association, University of Oklahoma, The Quadrangle Retirement Community, Lazard Global Healthcare Leadership Summit; Participation on the following: Village MD (External Advisory Board), Cellares (Board of Advisors), Notable (Occasional Advisor), HIEx Health Innovation Exchange Partnership (Advisory Board Member), JSL Health Fund (Advisory Board Member), WHO COVID-19 Ethics & Governance Working Group (Expert Advisory Member), Biden’s Transition COVID-19 Committee (Advisory Board Member), WHO (Special Advisor Board Member), Peterson Center on Healthcare (Advisory Board Member), Clarify Health (Advisor), FeelBetter (Advisory Board), Link Health Technologies (Advisor); Editorial Board Member of the Journal of the American Medical Association; Stock options for Cellares, Notable, Clarify, Korro, Link Health Technologies; JSL Health Fund (Invested in and no management fees); Oak HC/FT Venture Fund (Invested in fund); Council on Foreign Relations (Member), and Center for American Progress (Senior Fellow).
Funding
This work was supported by Janssen Research and Development LLC.
Craig Tendler, Patricia S. Hong, Conor Kane, Christa Kopaczynski, William Terry, and Ezekiel J. Emanuel